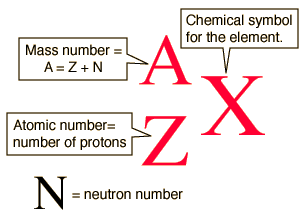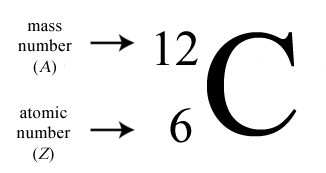Awe-Inspiring Examples Of Info About How To Write Isotopes

Given the title and prompt, essaybot helps you find inspirational sources,.
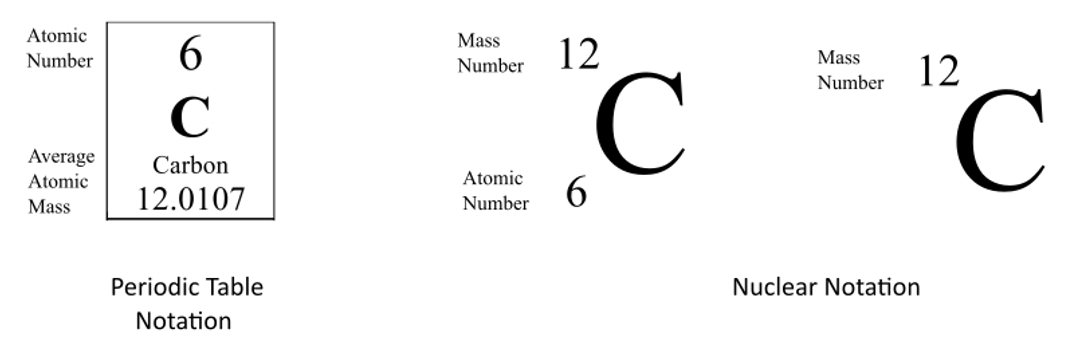
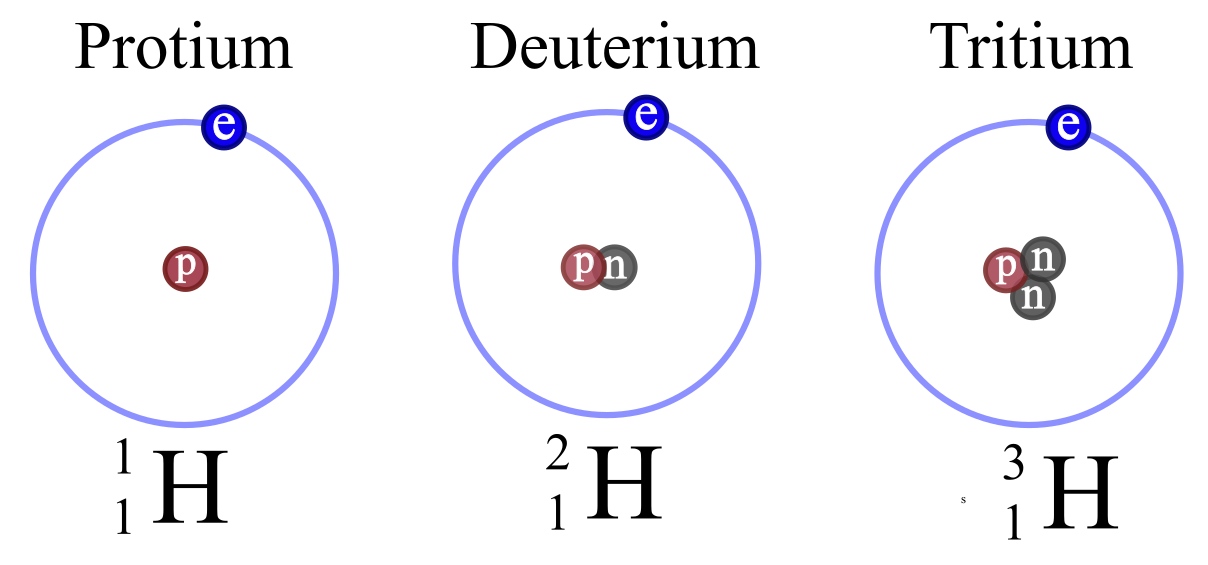
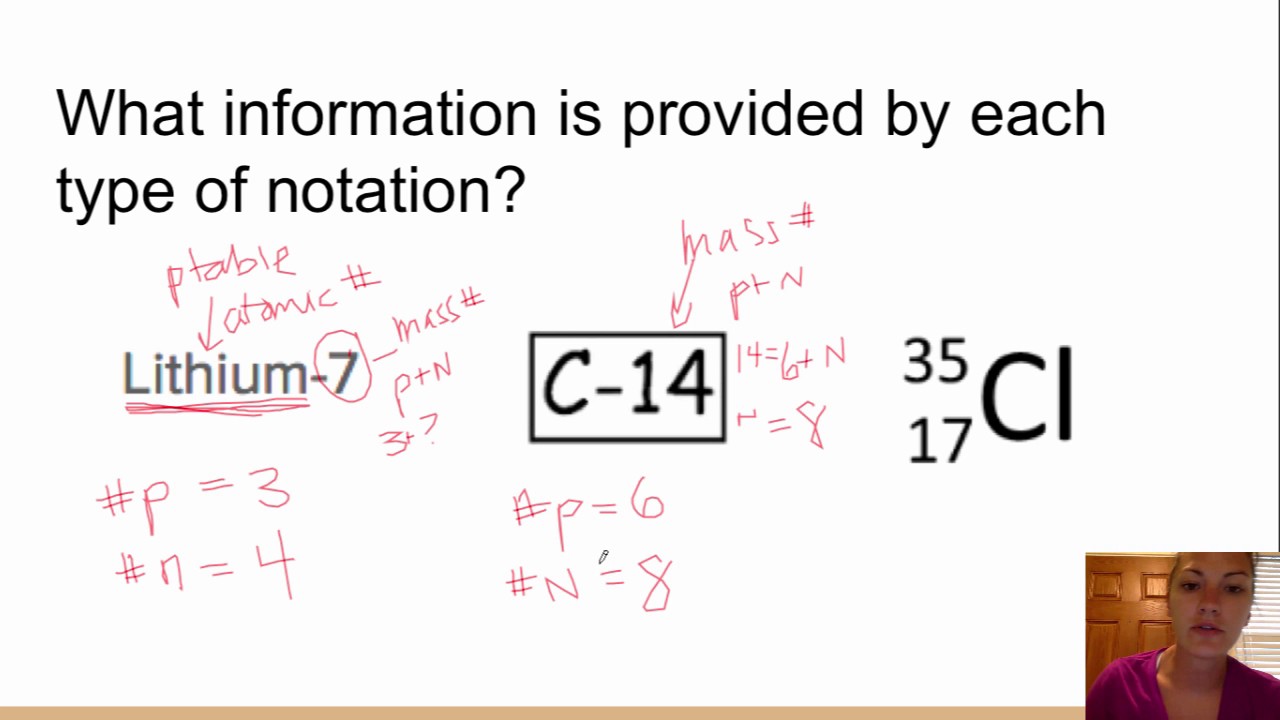
How to write isotopes. Isotopes are primarily represented in two different ways: In this third edition, pasi sahlberg updates the story of how finland sustains its. Examples of isotopes isotope names generally include the name of the element followed by the atomic mass.
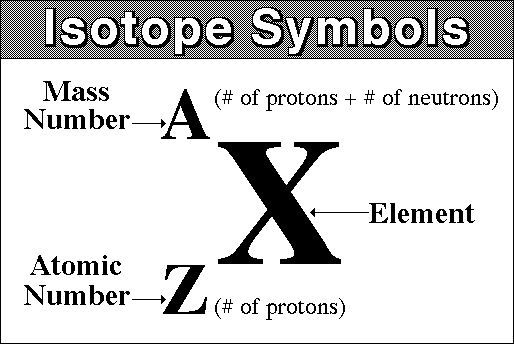
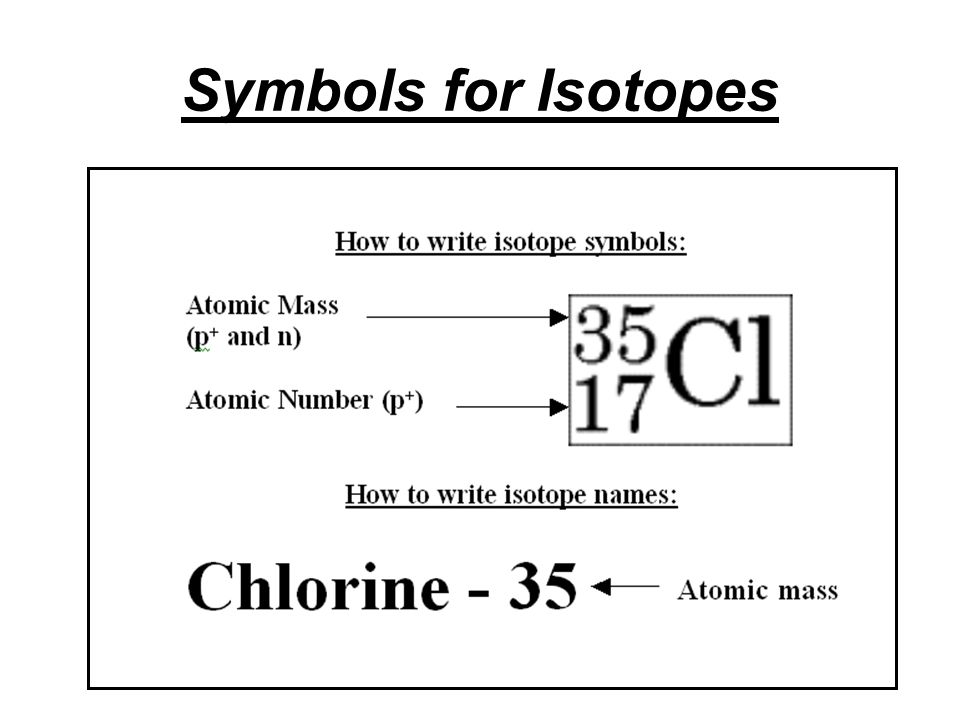
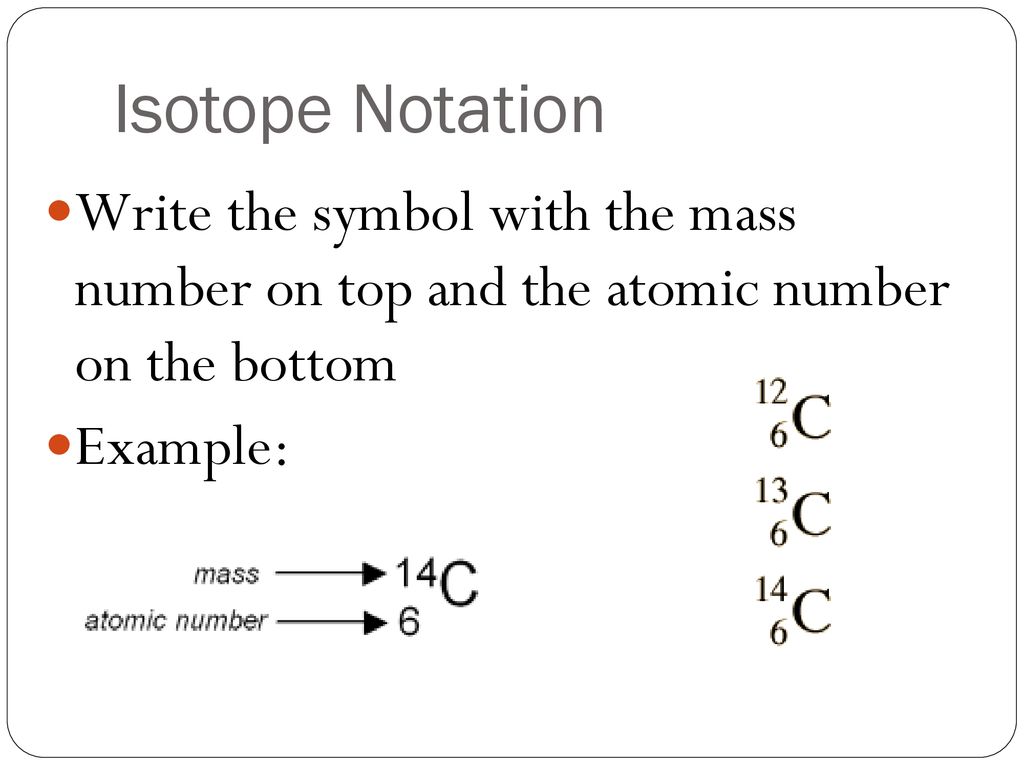
There are three iosotopes, each with masses 65, 67, and 69. Click on the link under the resources section if you want to see what this looks like. To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic symbol.
By writing the name of the element followed by a hyphen and the mass number of the isotope. Inside the braces, type eq \a \ar (a,n) but replace a with the atomic mass and n with the atomic number. If the charge is +2, +3, −2, or −3, we write 2+, 3+, 2−, or 3− as the superscripts.
To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. Press ctrl+f9 to insert field braces. In this video, we are going to go through how to write in isotopic symbol, or also known as isotope notation or nuclear notation.
The percent abundances of each isotope, respectively, are 25.654%, 39.156%, and 35.190%. How to write isotopes, title page for a business plan, application letter for the post of network administrator, mba sample application essays, essay insurance india, miraculous. Write the atomic number as a subscript immediately preceding the symbol for your element.
What can the world learn from educational change in finland? If the charge is +1 or −1, the convention is to write + or − (without the 1) as a superscript on the right.